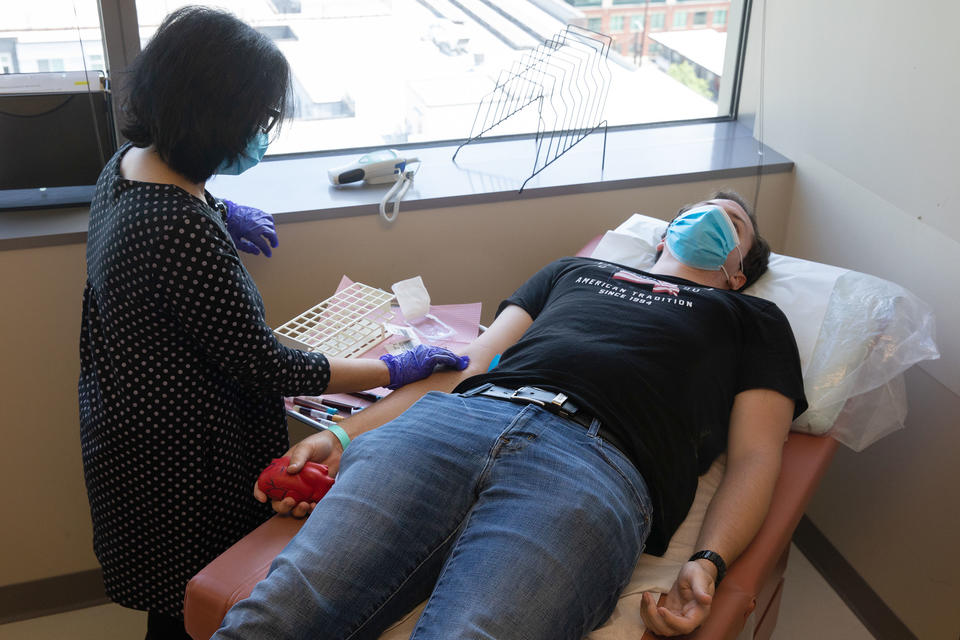
“I’m not sure that vein is as good on the left,” says Charles Jones, 21, looking at his right arm.
Jones, a University of Washington undergraduate, has really gotten to know his veins. He’s lying on an exam table at the University of Washington’s Harborview Medical Center in Seattle on June 10, preparing for yet another blood draw as a participant in a COVID vaccine clinical trial.
Some might wonder why he would want to take something experimental when there’s an abundance of Food and Drug Administration-approved vaccines available. Jones says contributing to science, and possibly improving his immune system beyond what the previously authorized vaccines can accomplish, is worth it. He may have a little buyer’s remorse, but not in a long-term sense.
“It might be more convenient to be vaccinated, but I think the benefits outweigh the negatives,” says Jones, who is studying psychology and business while working part time and serving as a teaching assistant.
Fortunately, Jones has good veins, Dr. Tia Babu tells him. She’s doing the blood draw after taking Jones’s vital signs at his first clinic visit since receiving his first dose of a second-generation coronavirus vaccine, one that researchers hope will provide lasting immunity to the virus and its variants.
For the past few months, Babu and Dr. Anna Wald, the UW principal investigator of the study, have been planning and conducting preliminary clinical trials of biotechnology company Gritstone’s CORAL vaccine. Gritstone’s “second generation” vaccine adds more defenses to the mix. It will — the manufacturer hopes — engage parts of the immune system some existing vaccines don’t reach. Researchers suspect the new vaccine will provide even broader immunity than those that have already received FDA approval and entered the market.
Despite missing out on some summer social activities and having no guaranteed level of immunity, trial participants believe more time under lockdown is worth it to move science forward. The doctors, both infectious disease physicians and faculty at the University of Washington School of Medicine, aim to enroll 40 unvaccinated people in the study. After just a few weeks of actively enrolling people, they have five participants already vaccinated; many others have reached out to sign up.
“It turns out that there are a lot of altruistic people in Seattle and in Washington state, who want to make a contribution,” Wald says.
Subjects at other CORAL trial locations received doses as early as March 30, but Seattle-based participants didn’t receive their vaccines until May at the earliest, when other COVID vaccines authorized for experimental emergency use were already available. With FDA-approved vaccines already available and a host of other vaccine trials long open, those involved in the Seattle CORAL study wondered whether they would find enough unvaccinated volunteers.
“I was just like, ‘Who’s going to do this when you can just go get vaccinated?’” says clinic manager Kirsten Hauge, manager of program operations at the UW Virology Research Clinic. “And I was surprised by how many people we got.”
Deciding to join the trial
Having moved to Seattle with her wife just before the pandemic set in, Nicole Rundlett was looking for a way to meaningfully give back when she heard about the vaccine trial.
A traveling sales manager, Rundlett isn’t used to spending so much time near and at home. She has kept busy by exercising, brewing beer, and filling a quarter of her 800-square-foot porch with potted plants, but she needed a community-oriented outlet. Having heard various conspiracy theories about vaccines, Rundlett — who comes from a family of medical workers, but who had never participated in a research study — thought she could lead by example. “I felt like I needed to do something to contribute,” she says.
Some trial members go to great lengths to test the vaccine. Jones lives in Poulsbo, and it takes him an hour or two by ferry to reach the clinic on Seattle’s First Hill. He has visited at least five times, beginning April 20.
Jones worried about whether he would need an approved vaccine to attend classes when the UW reopens in the fall, but liked the idea of having the stronger immunity CORAL may offer. He was also encouraged by his mom, who has cystic fibrosis, a condition that makes people more vulnerable to COVID. “She was so excited by the idea that I might be able to participate in something that would help people with CF,” he says.
So as other people started receiving proven vaccines, Rundlett and Jones agreed to bide their time.
How the vaccine works
Where the first generation of COVID vaccines have been shown to induce antibodies by using the virus’s spike protein, Gritstone’s second-generation CORAL vaccine adds more defenses to the mix. They’re experimenting not only with targeting different parts of the immune system, but using different types of vaccine delivery systems — adenovirus and mRNA — in the same patient.
Dr. Karin Jooss, Gritstone’s chief science officer, says the CORAL vaccine is designed to combat the virus if it manages to infect cells. Antibodies might not catch all of the virus you’re exposed to on its way into your body. This vaccine could boost T-cells — killer white blood cells — that can eliminate those infected cells. Gritstone’s goal is to prompt immune systems to respond quickly even a year or two after inoculation.
“We felt we needed to induce both arms of the immune system for best protection against the virus,” Jooss says. That cellular-level immunity may also protect a vaccine recipient against new variants, says Wald, the UW Medicine researcher leading the Seattle study.
Gritstone has been developing cancer vaccines for years. Since the pandemic set in, it has tried to apply that expertise to a coronavirus vaccine. Investigators at the four trial sites — UW, as well as Baylor, Emory and St. Louis universities — are working with Gritstone and the National Institute of Allergy and Infectious Diseases to test CORAL in the Phase 1 study.
Regardless of how well existing vaccines work, those already on the market are difficult to store and likely don’t exist in sufficient supply to help the whole world get the virus under control.
“There’s more than 7 billion people in the world, so we need more vaccines and more different types of vaccines to vaccinate everybody,” Wald says.
The trial
Getting into the trial is tough. Participants have to be very healthy, with low probability of exposure to coronavirus. Unvaccinated subjects are technically called “naive” subjects — ironic, as Babu and her colleagues make sure they’re fully informed.
“I’m a physician first,” Babu says. “I want them to understand everything before they decide,” she says.
Trial participants receive either two of the same vaccine types or one each of mRNA and adenovirus. They also receive versions of the vaccine with either just spike protein, or spike protein with additional proteins targeting the T-cell arm of the immune system.
While a few participants were enrolled at the beginning of May, the researchers voluntarily paused the trial for a few weeks while waiting for Centers for Disease Control and Prevention recommendations on the Johnson & Johnson vaccine, which also uses an adenovirus platform. When the trial restarted, the researchers allowed participants to elect not to get the adenovirus vaccine, but no one in the Seattle trial had chosen that option as of early June.
Rundlett says the trial has given her the confidence that, “yes, the medical community is absolutely doing this for the good of mankind and not some nefarious type of reason.”
She has had to forgo some tempting social opportunities as part of the trial, because she can’t assume she has immunity yet. She had planned to visit friends recently, but bowed out because they wanted to go to breweries. “I just decided, ‘Let’s wait another month or so.’ ”
Running out of unvaccinated subjects
It will be months before Rundlett or Jones know whether they’re protected against the coronavirus. The investigators plan to tell participants whether they’ve developed antibodies about three months after the last enrollee receives a second dose. Waiting isn’t easy, Rundlett says.
“I guess there’s still that unknown … so I am excited about seeing those results,” Rundlett says. “There are so many things I’m looking forward to. And I think my patience is wearing a little thin.”
The supply of unvaccinated volunteers — at least in the United States — is running low, though, and clinicians like Wald and Babu will soon have to account for that when testing new vaccines.
Gritstone originally anticipated starting a second study later this year, one that uses its vaccine as a booster dose in people who received first-generation vaccines, like those produced by Pfizer and Moderna. But this week the researchers received the go-ahead to weave that booster trial into this existing trial, giving some people one dose of the CORAL vaccine. They are hoping to start screening people soon for the booster trial and expect the crux of their study will focus on booster shots.
The contributions made by people like Rundlett and Jones, though, are vital to the research happening now, Babu emphasized.
“I think we need to continue to have volunteers like us willing to do this,” Rundlett says. “And I guess for me, I certainly feel that I would participate in other trials based on this experience.”